Nanox shares skyrocket on back of FDA approval for X-ray system
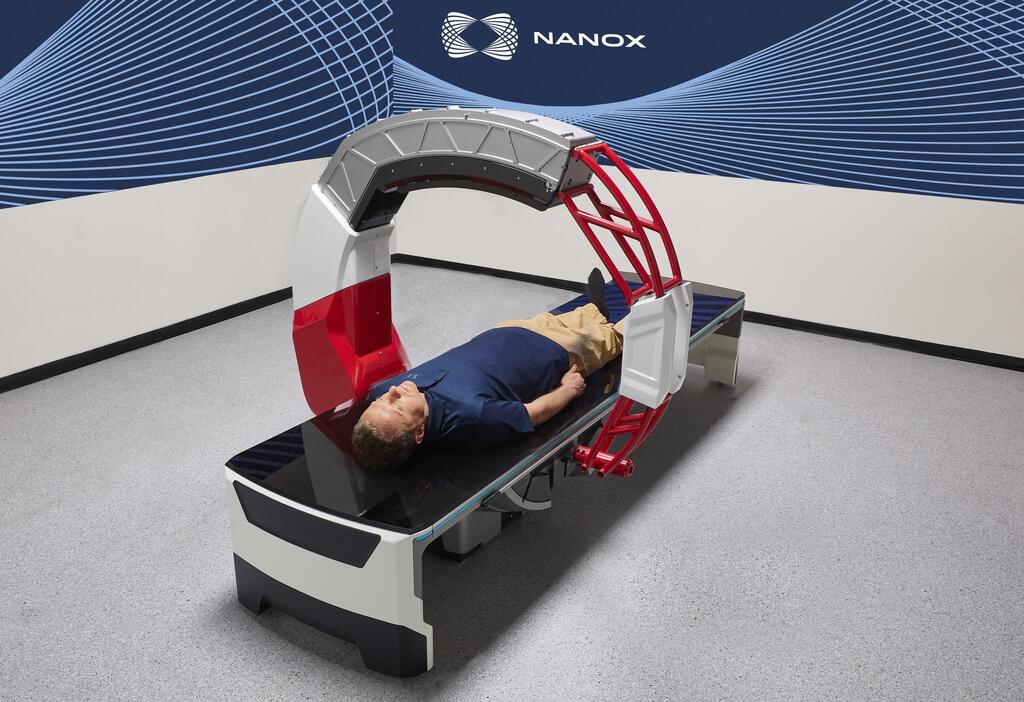
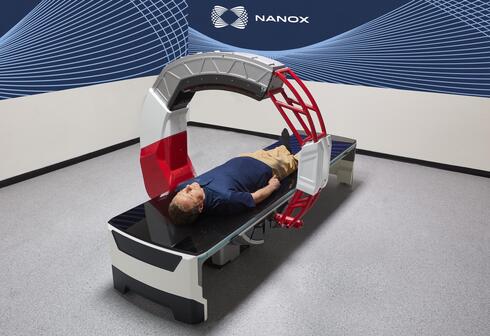
The Israeli medical imaging company received clearance for Nanox.ARC, a stationary X-ray system intended to produce tomographic images
Israeli medical imaging company Nanox saw its share price surge on Monday after announcing that it has received a 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the multi-source Nanox.ARC, including the Nanox.CLOUD, its accompanying cloud-based infrastructure.
Nanox.ARC is a stationary X-ray system intended to produce tomographic images of the human musculoskeletal system adjunctive to conventional radiography on adult patients.
The FDA cleared Nanox.ARC for use in professional healthcare facilities or radiological environments, such as hospitals, clinics, imaging centers, and other medical practices by trained radiographers, radiologists, and physicians.
The news resulted in Nanox shares experiencing a circuit breaker halt after soaring 75%. After trading resumed, the company’s stock was trading up by more than 50%. Nanox had previously experienced a 45% drop in its share price over the past year, but its market cap crossed the $500 million mark once more following Monday’s surge.
“Today’s milestone is a significant achievement as part of our commitment to make state-of-the-art medical imaging technology available for use in a wide array of professional healthcare facilities and other medical practices,” said Erez Meltzer, CEO of Nanox. “Our vision is that Nanox's innovative technology and approach not only have the potential to increase access to medical imaging, but also to shift healthcare from reactive to proactive—enabling early detection and prevention of diseases.”